Molecular Science and Engineering for Energy
We synthesize building blocks of materials, using principles from organic and polymer chemistry, assemble them to have tailored functionalities, and apply them to energy applications. Porous crystalline polymers, such as covalent organic frameworks (COFs), are our main foci. The versatility and modularity of the structures and, thus, tailored properties enable us to utilize them in energy storage/conversion devices, and catalysts. In particular, functional groups installed on porous and crystalline spaces maximize their properties, outperforming their conventional counterparts. Specifically, we develop organic-based solid electrolytes, interphase, and cathode materials for rechargeable batteries, such as lithium metal, lithium–sulfur, sodium metal, calcium-ion batteries, etc. Below are representative research directions.
Polymers with Higher Dimensions
We synthesize COFs and polymers using dynamic covalent chemistry to form crystalline, porous, and 2D (or 3D) materials with highly defined structures. The process typically involves condensation reactions, such as imine, boronate ester, or hydrazone formation, where monomers with specific geometries (e.g., linear, trigonal, or tetrahedral) are linked through reversible covalent bonds. These reactions are often conducted under solvothermal conditions, utilizing solvents like mesitylene or dioxane, or via mechanochemical methods, with catalysts or modulators to enhance reversibility and error correction. Precise control of reaction parameters—temperature, solvent composition, and reaction time—is critical to achieving high crystallinity and uniform porosity. The resulting COFs exhibit tunable pore sizes (1–5 nm) and high surface areas (up to 5000 m²/g), making them ideal for applications like ion transport, gas storage, catalysis, and sensing. The sophisticated monomer design and synthesis techniques enable tailored functionalities and robust frameworks for diverse applications.
Ion Conductive Frameworks
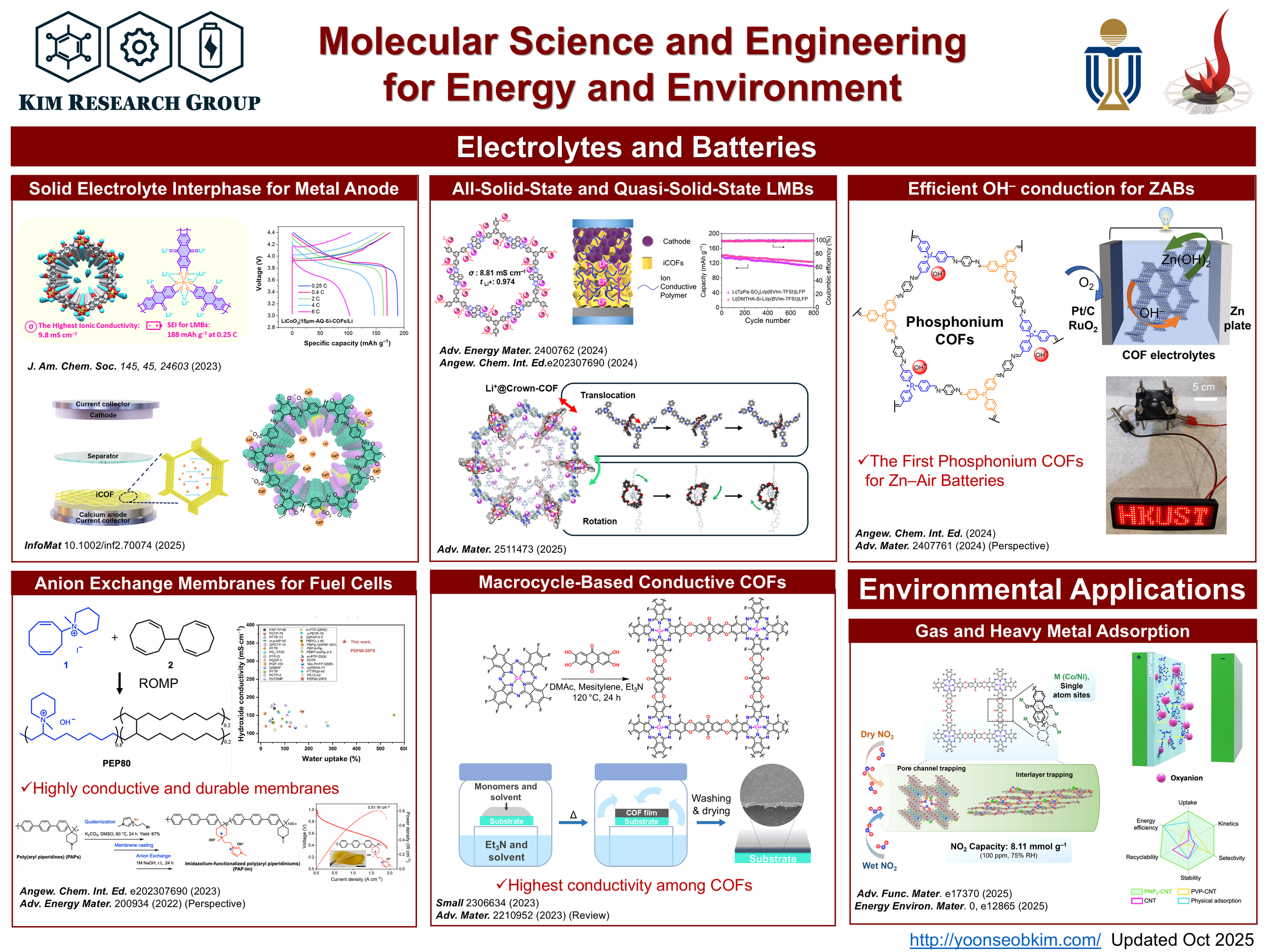
Ionic COFs (iCOFs) and polymers are synthesized through dynamic covalent chemistry, employing condensation reactions like imine or spiroborate formation to create crystalline, porous frameworks or amorphous polymers with tailored ion-conducting channels. Monomers with ionic groups (e.g., sulfonates or quaternary ammonium) are reacted under solvothermal or mechanochemical conditions, ensuring high surface areas and ordered pores for ion transport. Post-synthetic modification or addition of lithium salts enhances conductivity (up to 10⁻³ S/cm). These materials are utilized in solid-state batteries as electrolytes or electrode coatings, offering high ionic conductivity, thermal stability (up to 300°C), and mechanical robustness. Their tunable pore chemistry enables selective ion transport, improving battery capacity and cycling stability, making them promising for next-generation battery applications.
Solid-State Electrolytes
We utilize iCOFs as solid-state electrolytes in batteries by leveraging their ordered, porous structures to facilitate ion transport. COFs’ pores with lithium salts are functionalized to enhance ionic conductivity. The resulting COFs offer high thermal stability and mechanical strength, enabling their use as solid electrolytes. Counterions can be changed, so we use those them for various various battery types, such as lithium metal, sodium metal, calcium metal, zinc–air, calcium-ion, etc. They provide selective, rapid, and reliable ion transport, improving high battery capacity (e.g., >150 mAh/g) and cycling stability (>800 cycles) for efficient, safe energy storage.
Metal Anode Protection
Metal anodes, such as lithium and calcium, are inherently unstable due to the formation of ion-insulating passivation layers (e.g., CaF2, CaCO3, Li2CO3) during cycling, which hinders ion transport and leads to irreversible plating/stripping, causing capacity fade and dendrite growth. Ionic COFs (iCOFs) serve as protective layers to address these issues. iCOFs like anthraquinone-based silicate COFs and sulfonate-based TpPa-SO3Ca0.5 offer high ionic conductivity (10⁻⁴–10⁻³ S/cm) and selective ion transport through ordered nanochannels. These properties promote uniform metal deposition, suppress side reactions, and prevent dendrite formation. By stabilizing the anode-electrolyte interface, iCOFs enable reversible plating/stripping over hundreds of cycles, significantly enhancing battery stability, energy density, and cycling performance in calcium and lithium metal batteries.